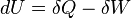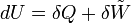First Law of Thermodynamics
The first law of thermodynamics, an expression of the principle of conservation of energy, states that energy can be transformed (changed from one form to another), but cannot be created or destroyed. It is usually formulated by saying that the change in the internal energy of a system is equal to the amount of heat supplied to the system, minus the amount of work done by the system on its surroundings.
The first law of thermodynamics says that energy is conserved in any process involving a thermodynamic system and its surroundings. Frequently it is convenient to focus on changes in the assumed internal energy (U) and to regard them as due to a combination of heat (Q) added to the system and work done by the system (W). Taking dU as an infinitesimal (differential) change in internal energy, one writes
where δQ and δW are infinitesimal amounts of heat supplied to the system and work done by the system, respectively. Note that the minus sign in front of δW indicates that a positive amount of work done by the system leads to energy being lost from the system.
Depending on discipline, an alternative convention may be adopted for the "work" under consideration, leading to:
where  is the work done on the system by the surroundings.[1]
is the work done on the system by the surroundings.[1]
When a system expands in a quasistatic process, the work done on the system is − PdV whereas the work done by the system while expanding is PdV. In any case, both give the same result when written explicitly as:
Work and heat are due to processes which add or subtract energy, while U is a particular form of energy associated with the system. Thus the term "heat energy" for δQ means "that amount of energy added as the result of heating" rather than referring to a particular form of energy. Likewise, "work energy" for δw means "that amount of energy lost as the result of work". Internal energy is a property of the system whereas work done and heat supplied are not. A significant result of this distinction is that a given internal energy change (dU) can be achieved by, in principle, many combinations of heat and work.
Informally, the law was first formulated by Germain Hess via Hess's Law, and later by Julius Robert von Mayer[2]
Zeroth Law of Thermodynamics
A system is said to be in thermal equilibrium when its temperature does not change over time. Let A, B, and C be distinct thermodynamic system or bodies. The zeroth law of thermodynamics can then be expressed as
"If A and C are each in thermal equilibrium with B, A is also in thermal equilibrium with C."
The preceding sentence asserts that thermal equilibrium is a Euclidean relation between thermodynamic systems. If we also grant that all thermodynamic systems are (trivially) in thermal equilibrium with themselves, then thermal equilibrium is also a reflexive relation. Relations that are both reflexive and Euclidean are equivalence relations. One consequence of this reasoning is that thermal equilibrium is a transitive relation between the temperature T of A, B, and C:
- If T (A) = T(B)
- And T (B) = T(C)
- Then T (A) = T(C).
if two bodies are in thermal equilibrium with a third body,then the two bodies are in thermal equilibrium with each other